How to combat fraud and noncompliance in your Life Science organization
Fraud has been a hot topic lately, and for good reason. According to the Association of Certified Fraud Examiners’ 2016 Global Fraud Study, the median loss for occupational fraud is $150,000, while 23.2% of occupational fraud cases cause losses of $1 million or more.
While business leaders may think it won’t happen to them, the same study found that the typical organization loses five percent of its annual revenue to fraud. When you consider the likelihood of fraud, along with increasing scrutiny from government regulations, pharmaceutical, biotech and medical device organizations especially face harmful threats from all sides.
Vulnerability in Life Sciences
The costs of occupational fraud and noncompliance can impact organizations of any size and in any industry. But the nature of the Life Sciences industry makes it especially vulnerable to attack. Consider these factors:
- Increasingly complex regulatory environment: From the Sunshine Act to Transparency Reporting, organizations have a myriad of regulations they must comply with or face serious legal ramifications. Additionally, most organizations are incorporating new technology to improve patient outcomes, but regulations for these new technologies are not clearly defined. As such, organizations can be left guessing on what is in compliance and what is not.
- Ineffective global view: Great access to international markets has driven globalization into the industry. But with global operations comes the need for greater integration across the company and understanding of global compliance. Employee spend information in disparate systems makes it difficult to get a clear global view of all company spend, plus makes it easier for fraudsters to cover up their schemes. An effective global view allows organizations to detect spend patterns and red flags so they can see spending clearly and manage and prevent fraud and noncompliance proactively.
- Overlooked valuable data: Life Sciences organizations traditionally utilize historical data for reporting purposes. But few have embraced data to spot issues, identify trends and prioritize auditing resources. Capturing and connecting all data allows companies to proactively prevent, manage and detect occupational fraud, ensure regulatory compliance and obtain additional actionable insights to make smarter business decisions.
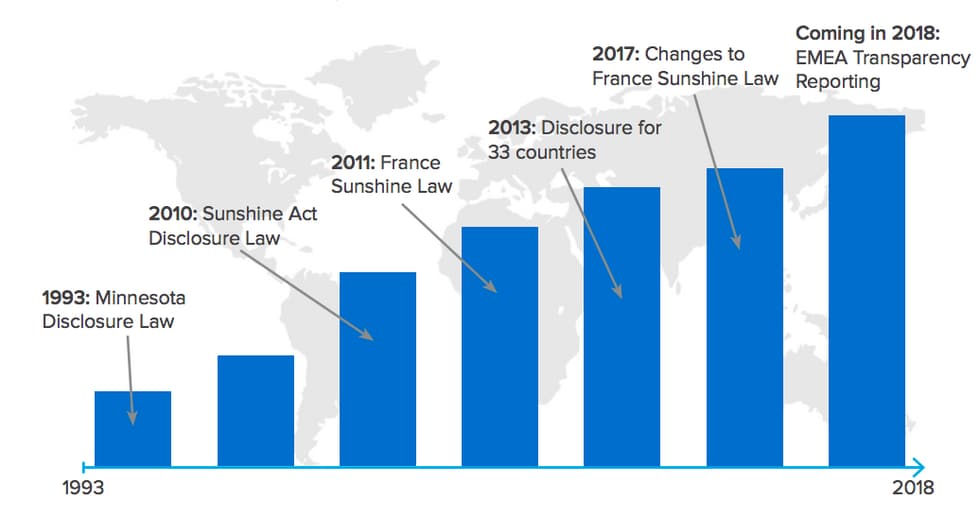
Figure 1: Increase in global regulations
Protecting your organization with a digital trench
As a member of a Life Sciences organization, you may be quick to assume that instances from travel and expense (T&E) fraud may be few and far between. The truth is, asset misappropriation is by far the most common form of occupational fraud, occurring in more than 83% of cases. Examples of asset misappropriation include employees embezzling company assets and padding T&E claims. If you have multiple instances of fraud within a year, these instances can absolutely add up to more than just a few dollars. Often, discovering a red flag of fraud in T&E can be the thread that unravels an entire fraud scheme impacting your organization.
Embracing an effective T&E policy and the right spend management technology can help your organization build a digital trench to protect your bottom line—both from internal fraud and external regulatory violations.
In order to help to ensure your T&E program provides the protection your organization needs, your organization should take steps to:
- Mandate a corporate card program to auto-import expenses into your expense management system.
- Adopt systems that allow employees to submit expenses for preapproval.
- Use a third-party audit service to maintain credibility.
- Require proper data entry for expense reports: set audit rules, require receipt type (paper, electronic, automated) and itemized receipt requirements type.
- Share data and package it for key stakeholders within your organization to highlight problem areas.
Lasting change in spend and beyond
Correctly managing compliance, fighting fraud and meeting your regulatory obligations takes a lot of work—and time. And likely, more time and energy than your company can manage alone.
Understanding what makes your Life Science organization vulnerable to attack is the first step. It’s even more important that you pinpoint problem areas early and establish an efficient end-to-end spend management process to keep you in control and your company protected. By taking these steps now, you can help shift your culture in ways that lead to lasting improvements in spend and beyond.
Learn more about how Concur helps with Pharma, Biotech and Medical Device organizations build their own digital trench.